|
3 讨论
以往的研究表明,视杆外节膜盘含有大量的磷脂[4],磷脂的β位上有丰富的花生酸类的前体。磷脂酶A2(phospholipase a2,PLA2)可催化β键的断裂并释放出AA。AA经过环氧酶(cyclo-oxygenase)作用而产生前列腺素I2(prostaglandin I2,PGI2)和PGE2。PGI2的性质极不稳定,在短时间内转化成6-keto-PGF1α,所以6-keto-PGF1α可以间接反映PGI2的水平。本实验表明,光照后两组视网膜的PGE2和6-keto-PGF1α的含量均有增加,只是增加的程度不同。这种PG合成的增加可能是引起视网膜自由基含量升高的因素之一。另有实验发现,光照后6小时视网膜蛋白激酶C(protein kinase C,PKC)的活性升高[3],与此同时出现的PGE2与6-keto-PGF1α含量增加可能有一定的关系。因为PKC的激活有Ca2+的增加,后者可激活PLA2。近期的研究表明,PKC通过对lipocortin(脂调素或脂皮素)的磷酸化,使其丧失对PLA2的抑制作用,从而引起PG的合成增加[5]。视网膜光化学损伤中AA代谢明显增强所产生的自由基以及AA衍生物的扩张血管和吞噬细胞趋化作用,在诱发或加重视网膜光化学损伤过程中起到一定的作用。
视网膜光性损伤的药物防治研究,大多是从抗氧化和清除自由基两条路径考虑,如用维生素 e等。此外,flunarizine和皮质类固醇也被同类实验采用,前者可降低Ca2+的水平,而后者的作用认为是与稳定细胞膜有关[6-9]。虽然在解释上述药物的疗效时,大部分作者并未提及对PG合成的影响,但事实上维生素 e及其它酚类抗氧化剂均能抑制AA的过氧化反应;flunarizine可阻滞Ca2+水平升高,影响PLA2的活性;皮质类固醇是目前最佳的AA代谢抑制剂,它可诱导lipocortin的合成,lipocortin与PLA2结合使其丧失激发AA代谢的能力。本实验结果显示,DXM组在光照后6小时和1,3天视网膜的PGE2和6-keto-PGF1α含量明显低于对照组,表明DXM可抑制视网膜光化学损伤中AA的代谢过程,对于7,14天该组2种PG水平升高,可能与DXM的用药期和半衰期较短有关。通过阻止PG的生成,DXM可能对视网膜光化学损伤起到防护作用。
表1 大鼠视网膜6-keto-PGF1α的含量(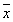 ±s)(pg/mg) ±s)(pg/mg)
Tab. 1 levels of the 6-keto-PGF1α in rat retina (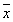 ±s)(pg/mg) ±s)(pg/mg)
| Groups |
Before
light exposure |
After light exposure |
| 6h |
1d |
3d |
7d |
14d |
| Control |
1.96±0.30 |
4.68±0.69* |
7.50±0.57* |
10.40±0.71* |
8.88±0.99* |
3.69±0.77* |
| DXM |
2.10±0.40 |
2.50±0.59▲ |
4.68±0.81+▲ |
6.87±1.10+▲ |
8.41±0.95+ |
4.29±0.58+ |
* Significantly different(P<0.01) from the control group before light exposure. + Significantly different(P<0.01) from the DXM group before light exposure. ▲ Significant differences(P<0.01) between DXM and Control groups at same time
表2 大鼠视网膜PGE2的含量(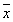 ±s)(pg/mg) ±s)(pg/mg)
Tab. 2 levels of the PGE2 in rat retina (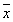 ±s)(pg/mg) ±s)(pg/mg)
| Groups |
Before
light exposure |
After light exposure |
| 6h |
1d |
3d |
7d |
14d |
| Control |
13.33±1.93 |
37.50±2.75* |
48.06±4.04* |
81.90±4.89* |
68.26±3.85* |
34.94±4.23* |
| DXM |
11.45±1.90 |
20.60±4.28+▲ |
37.36±3.34+▲ |
54.85±4.57+▲ |
70.08±3.57+ |
38.51±2.85+ |
* Significantly different(P<0.01) from the control group before light exposure. + Significantly different(P<0.01) from the DXM group before light exposure. ▲ Significant differences(P<0.01) between DXM and Control groups at same time
本课题受国家自然科学基金资助,基金号39170768
4 参考文献
[1] Kuehl FA,Humes JL,Egan PW,et al.Role of prostaglandin endoperoxide in inflammatory processes.Nature,1977,265:170-173.
[2] Zurier RB.Prostaglandins and inflammation.In:Curtis-Prir pB,ed.Prostaglandins:biology and chemistry of prostaglandins and related eicosanoids.London:Churchill Livingstone,1988.595-607.
[3] 张军军,严密,张敏.视网膜光化学损伤中的蛋白激酶C.中华眼底病杂志,1997,13:78-80.
[4] Stinson AM,Wiegand RD,Anderson RE.Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes.Exp eye Res,1991,52:213-218.
[5] Boneh A,Shohami E,Brenner T.Differential effects of phorbol
myristate acetate and dexamethasone on protein kinase C activity
and eicosanoids production in cultured rat astrocytes.J Neurosci
res,1993,34:629-634.
[6] Fu J,Lam TT,Tso MOM.Dexamethasone ameliorates retinal photic injury in albino rats.Exp Eye Res,1992,54:583-594.
[7] Li J,Edward DP,Lam TT,et al.Amelioration of retinal photic injury by a combination of flunarizine and dimethylthiourea.Exp Eye Res,1993,56:71-78.
[8] Ham WT,Mueller HA,Ruffolo JJ,et al.Basic mechanisms underlying the production of photochemical lesions in the mammalian retina.Curr Eye res,1984,3:165-174.
[9] Parver LM,Auker CR,Fine BS,et al.Dexamethasone protection against photochemical retinal injury.Arch Ophthalmol,1984,102:772-777.
(收稿:1998-11-25 修回:1999-01-10) 上一页 [1] [2] |
